In particular, ‘metabolic dysfunction-associated SLD’ (MASLD for short) can develop due to adverse lifestyle factors such as a high-energy diet and little exercise. It already affects around a third of all people worldwide. Initially, MASLD has no pathological effects, but it can develop into inflammation of the liver. In the long term, this may lead to liver cirrhosis, liver failure or even liver cancer. There is no substitute procedure that can take over the function of the liver in the long term, such as dialysis for kidney failure. Those affected are at high risk and may only be cured by a liver transplant.
In addition, people with MASLD have an increased risk of developing type 2 diabetes mellitus or dying from cardiovascular diseases. Obesity favours MASLD, but not all obese people are affected. And conversely, slim people can also develop the disease.
The molecular causes of the development of MASLD are not fully understood. A team of researchers from HHU, the DDZ (Leibniz Centre for Diabetes Research at HHU), Düsseldorf University Hospital (UKD) and Forschungszentrum Jülich (FZJ) have now discovered an important aspect that explains how MASLD develops.
The leading role is played by windows (Latin: fenestrae) in the endothelial cells of blood vessel, through which substances are exchanged between liver cells and blood. The liver uses these tiny windows to release excess fat particles into the adipose tissue via the bloodstream. The researchers discovered that these windows are closed by a mechanism in which the signalling molecule SEMA3A (semaphorin-3A) plays the central part. This molecule is produced in blood vessels when they are overly exposed to the saturated fatty acid “palmitic acid“.
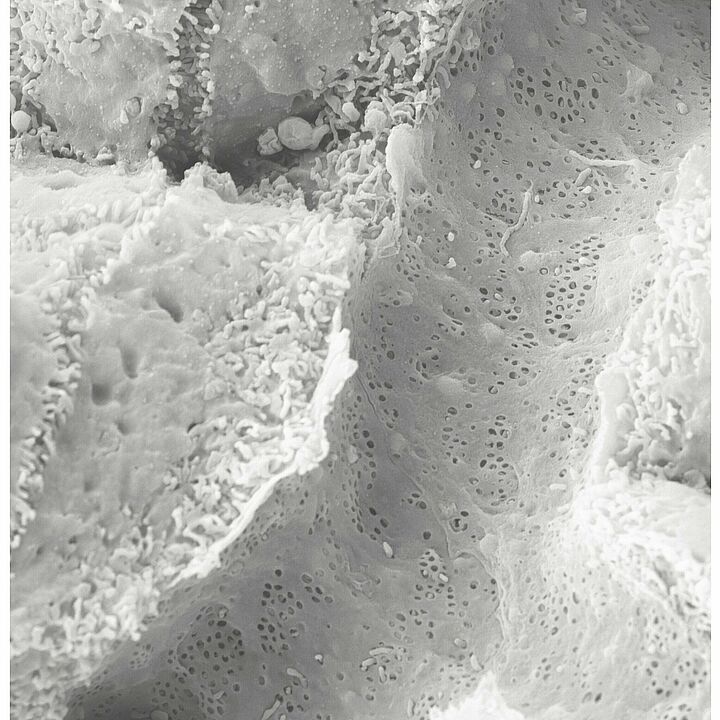
Sydney Balkenhol from the Institute of Metabolic Physiology at HHU and the DDZ, first author of the study now published in Nature Cardiovascular Research, points to a discovery made by the team using scanning electron microscopy: The “windows” in the smallest blood vessels of the liver were also closed in mice with fatty liver and type 2 diabetes mellitus.
Dr Daniel Eberhard, the other first author, adds: “We were also able to reverse the effect. By inhibiting the signalling molecule, we could defat the liver and thus improve its function again."
Corresponding author Dr Eckhard Lammert, Professor and Head of the Institute of Metabolic Physiology at HHU and the Institute of Vascular and Islet Cell Biology at the DDZ, hopes that the discoveries will also lead to a therapeutic approach for humans in the long term: “It may be possible to use the SEMA3A signalling molecule we identified to prevent MASLD and its consequences at an early stage. However, we first need to investigate the processes in humans in detail."
Original publication
Daniel Eberhard, Sydney Balkenhol, Andrea Köster, Paula Follert, Eric Upschulte, Philipp Ostermann, Philip Kirschner, Celina Uhlemeyer, Iannis Charnay, Christina Preuss, Sandra Trenkamp, Bengt-Frederik Belgardt, Timo Dickscheid, Irene Esposito, Michael Roden & Eckhard Lammert, Semaphorin-3A regulates liver sinusoidal endothelial cell porosity and promotes hepatic steatosis, Nature Cardiovascular Research (2024). DOI: 10.1038/s44161-024-00487-z
https://www.nature.com/articles/s44161-024-00487-z